Matt Stefely. 9 years ago. The two oxygens are both partially negative, this is what the resonance structures tell you! While both resonance structures are chemically identical, the negative charge is on a different oxygen in each. This is important because neither resonance structure actually exists, instead there is a hybrid.
Ultimate Guide to GCE A Levels H2 Biology | Biology H2 – GCE A Level | Thinkswap
Step by step Solved in 2 steps with 1 images SEE SOLUTION Check out a sample Q&A here Knowledge Booster Learn more about Theories of Bonding Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.
Source Image: illustrationart.blogspot.com
Download Image
Objectives After completing this section, you should be able to use the concept of resonance to explain structural features of molecules and ions. understand the relationship between resonance and relative stability of molecules and ions. Rules for Drawing and Working with Resonance Contributors
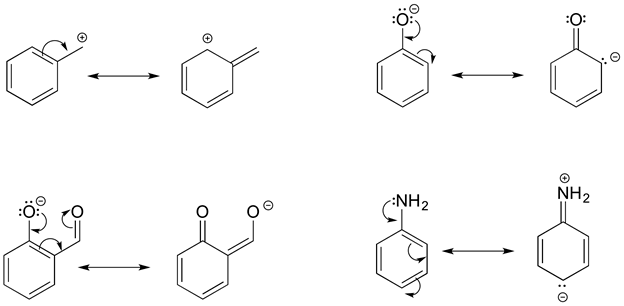
Source Image: chem.libretexts.org
Download Image
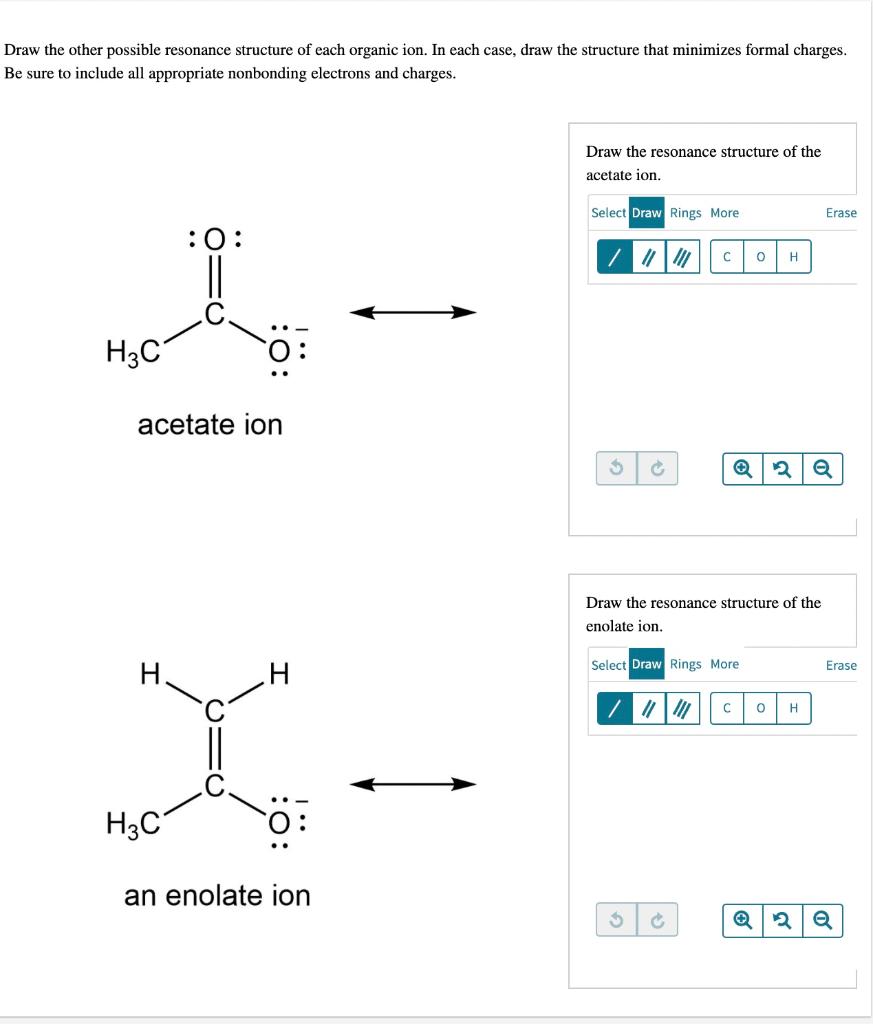
Solved Draw the other possible resonance structure of each | Chegg.com In this example problem, we draw the Lewis structure for phosphate which minimizes formal charge; there are multiple resonance structures.
Source Image: chegg.com
Download Image
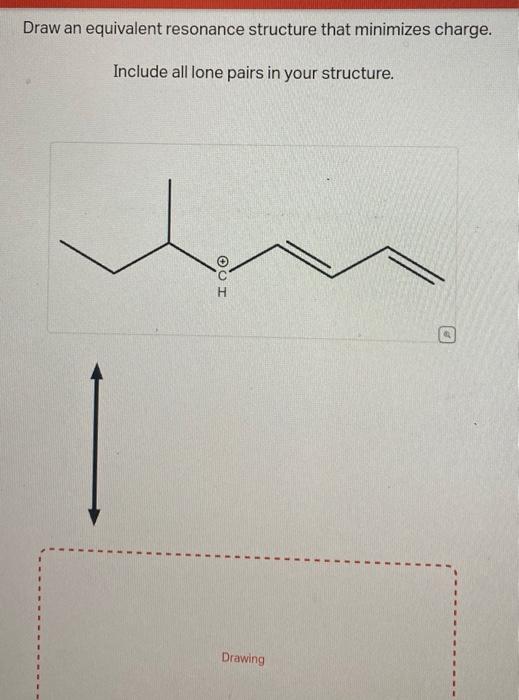
Draw An Equivalent Resonance Structure That Minimizes Charge
In this example problem, we draw the Lewis structure for phosphate which minimizes formal charge; there are multiple resonance structures. Evaluating The Resonance Forms Of Ethene (“Ethylene”) The simplest molecule with a π bond is ethene. If we draw a resonance structure for it, we can move the π bond to the lone pair of one of the end carbons (doesn’t matter which one) to give a carbocation and a lone pair. This is a “legal” resonance form, since we’re not breaking
Solved Draw an equivalent resonance structure that minimizes | Chegg.com
Exercise 2.6.1 2.6. 1. Draw the resonance contributors that correspond to the curved, two-electron movement arrows in the resonance expressions below. Then identify the type of resonance motion in each structure below. Answer. Exercise 2.6.2 2.6. 2. In each resonance expression, identify the type of resonance motion. Equivalent Lewis Structures Example – YouTube

Source Image: youtube.com
Download Image
Energies | Free Full-Text | Advanced Electric Vehicle Fast-Charging Technologies Exercise 2.6.1 2.6. 1. Draw the resonance contributors that correspond to the curved, two-electron movement arrows in the resonance expressions below. Then identify the type of resonance motion in each structure below. Answer. Exercise 2.6.2 2.6. 2. In each resonance expression, identify the type of resonance motion.
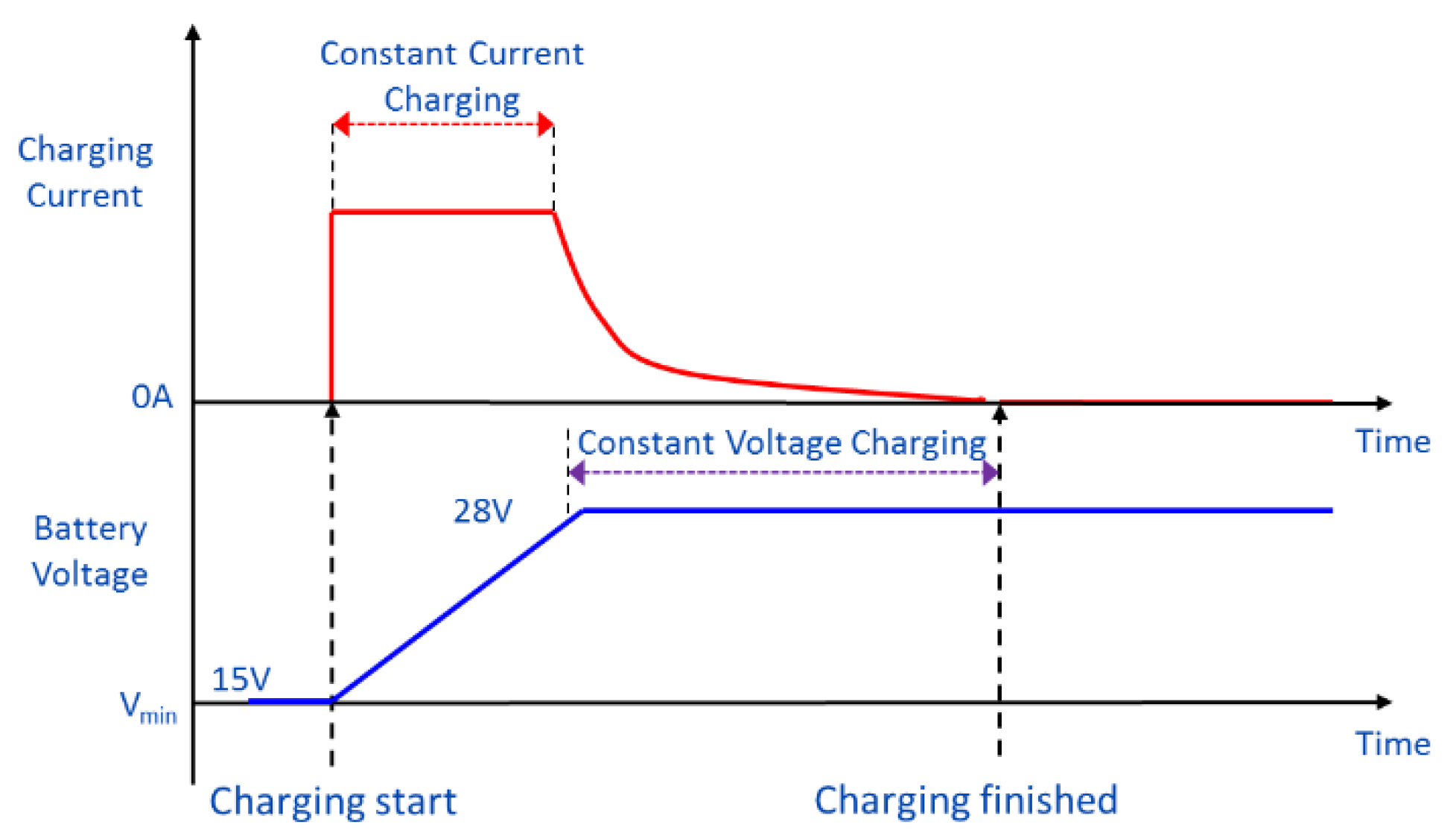
Source Image: mdpi.com
Download Image
Ultimate Guide to GCE A Levels H2 Biology | Biology H2 – GCE A Level | Thinkswap Matt Stefely. 9 years ago. The two oxygens are both partially negative, this is what the resonance structures tell you! While both resonance structures are chemically identical, the negative charge is on a different oxygen in each. This is important because neither resonance structure actually exists, instead there is a hybrid.

Source Image: thinkswap.com
Download Image
Solved Draw the other possible resonance structure of each | Chegg.com Objectives After completing this section, you should be able to use the concept of resonance to explain structural features of molecules and ions. understand the relationship between resonance and relative stability of molecules and ions. Rules for Drawing and Working with Resonance Contributors
Source Image: chegg.com
Download Image
NMR Analysis, Processing and Prediction: 2009 Minor resonance structures are all the resonance contributors that are higher in energy than the lowest-energy contributor. For example, we can draw three possible contributors for formamide, HCONH₂. We have to decide which of these is the lowest-energy form. That one will be the major contributor. All the others will be minor contributors.

Source Image: nmr-analysis.blogspot.com
Download Image
Basic must-follow criteria to be considered during the design and construction of machine foundation | Strukts In this example problem, we draw the Lewis structure for phosphate which minimizes formal charge; there are multiple resonance structures.

Source Image: strukts.com
Download Image
Solved Draw an equivalent resonance structure that minimizes | Chegg.com Evaluating The Resonance Forms Of Ethene (“Ethylene”) The simplest molecule with a π bond is ethene. If we draw a resonance structure for it, we can move the π bond to the lone pair of one of the end carbons (doesn’t matter which one) to give a carbocation and a lone pair. This is a “legal” resonance form, since we’re not breaking
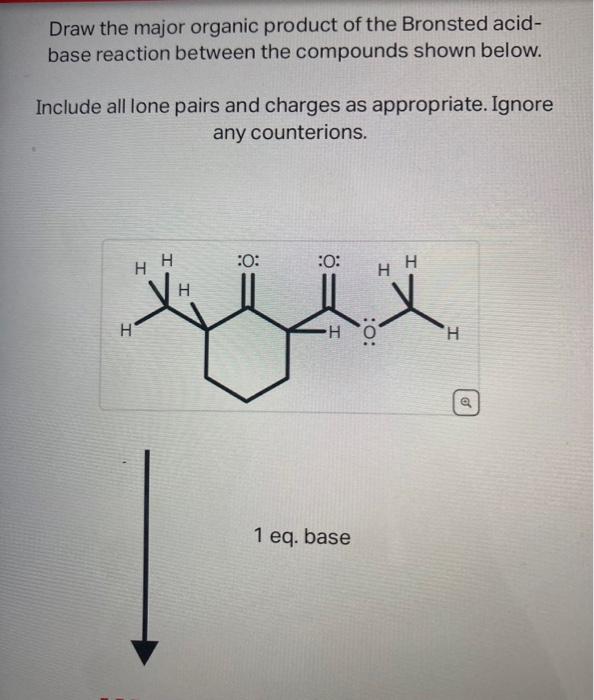
Source Image: chegg.com
Download Image
Energies | Free Full-Text | Advanced Electric Vehicle Fast-Charging Technologies
Solved Draw an equivalent resonance structure that minimizes | Chegg.com Step by step Solved in 2 steps with 1 images SEE SOLUTION Check out a sample Q&A here Knowledge Booster Learn more about Theories of Bonding Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.
Solved Draw the other possible resonance structure of each | Chegg.com Basic must-follow criteria to be considered during the design and construction of machine foundation | Strukts Minor resonance structures are all the resonance contributors that are higher in energy than the lowest-energy contributor. For example, we can draw three possible contributors for formamide, HCONH₂. We have to decide which of these is the lowest-energy form. That one will be the major contributor. All the others will be minor contributors.